Overview
CompliantPro Quality Management System Software is a tool that ensures a company’s quality assurance requirements are documented in a single, integrated system which demolishes silos of information managed as point solutions. CompliantPro allows an organization to manage its compliance and regulatory requirements beyond just document control. Key areas include Environmental Health & Safety, Enterprise Risk Management, Training & Qualifications, Audits, and customer / supplier management.
CompliantPro Functional Capabilities
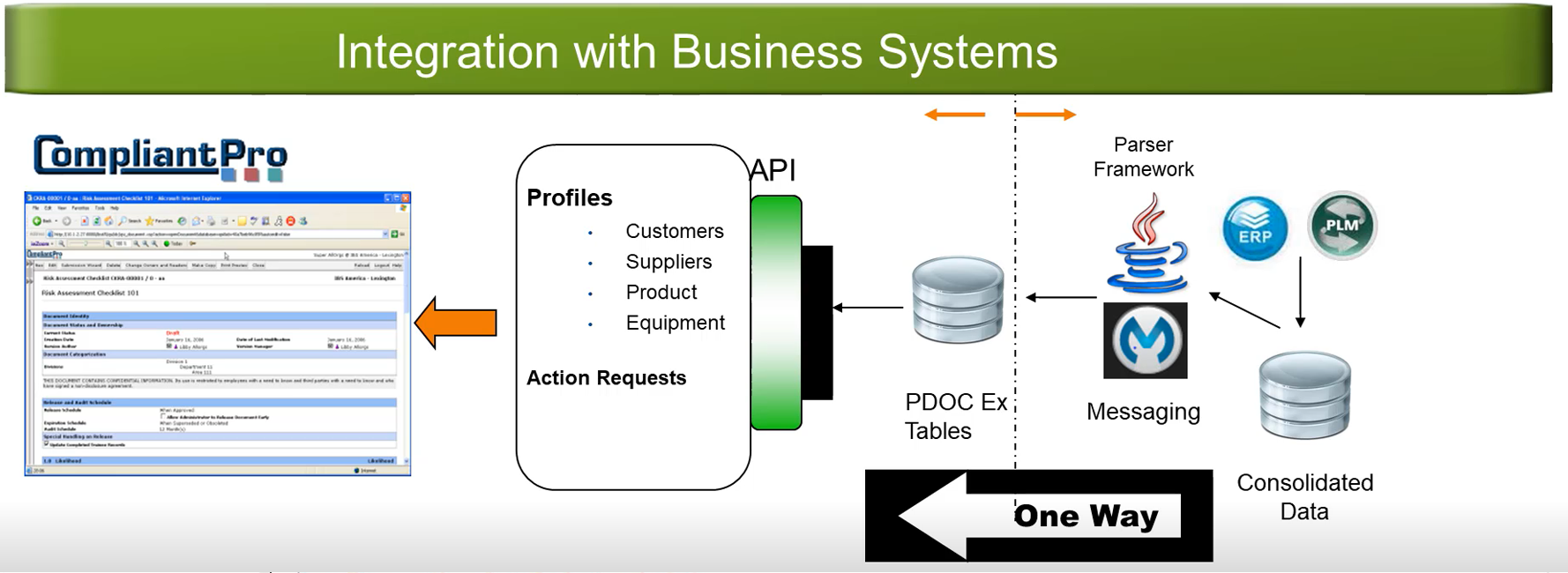
- Integration Capability
- Multilingual
- Reporting & Analytics
- Dashboards
- Escalation
- Action Management
- Back-ups / Delegates
- Configurable
CompliantPro Quality Management System Software has built-in global features that will allow organizations to better manage their global sites QM requirements through one system. These features include Organizations, Rules-based Configurable Workflows, Cloning, Escalation, Categorization, Approval Workflows, Pre-Configured Dynamic Reports, Content Structured via Templates, Saved Searches, Read Restrictions, Reporting Tables for BI Tool Access, Numbering of Documents, and Roles-based Security.
Document Control
- Document Ownership
- Collaboration Process (Optional)
- Final Approval Process via
- Electronic Signature
- Automatic Release and Control of Versions
- Delayed Release Capability
- Temporary Document Capability
- Automatic Archiving of Previous Versions
- Automatic Audit Reminders
- Integration with Training and Qualification
- Integration with Assessments
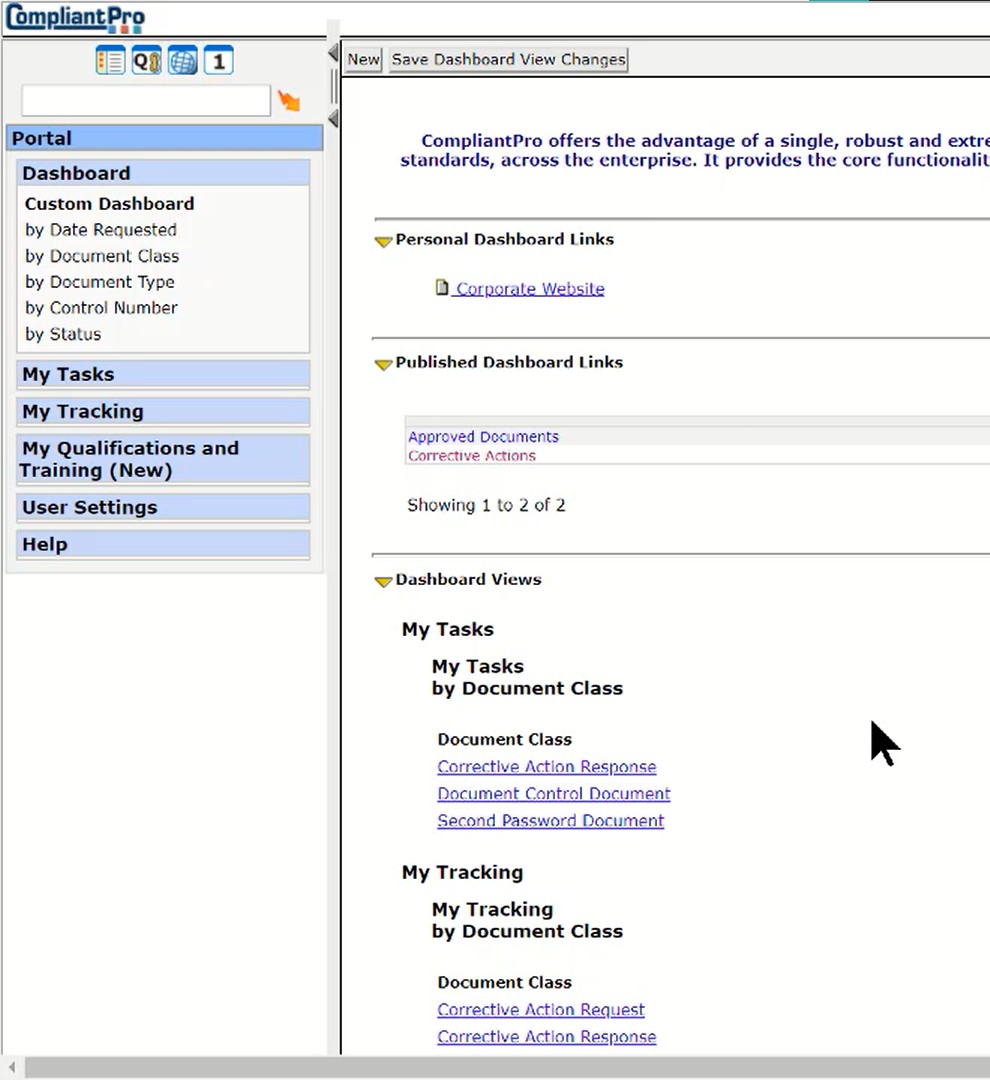
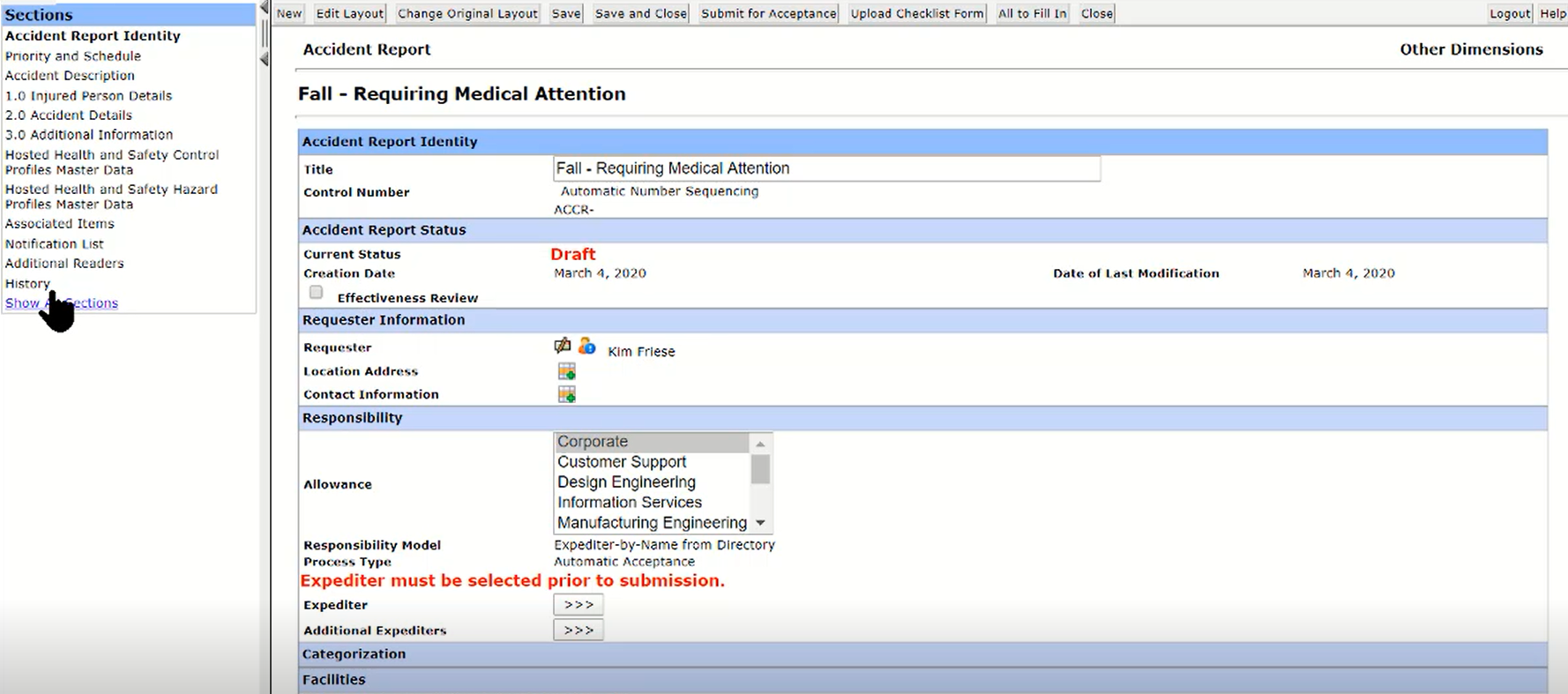
Personal Dashboard and Reports- Quick Access to Most Relevant Information
- My Tasks
- My Tracking
- Personal Links
- Published Links
- Dynamic Pre-Configured Reports
Features of CompliantPro
- Document Control
- Training and Qualification
- Enterprise Risk Management
- CAPA Management
- Meeting Management
- Quality Planning & Objectives
- Audit Management
- Customer Surveys
- Supplier Management
- Change Management
- Nonconformances / Waivers
- Complaint Management
- Calibration Management
- Environmental/Health/Safety Management
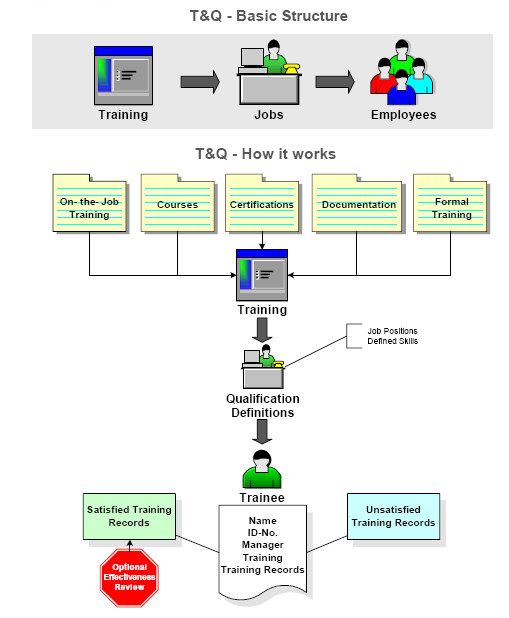
Training and Qualifications - Visibility to who can perform what assignment
- Track job qualifications and training requirements
- Track employee and non-employee training information
- Certification and renewal course reminders
- Quizzes and Effectiveness Reviews
- Integration with Document Control
- Self-acknowledgement of training
- Mass scheduling
- Mass updating
- Escalation
CAPA (Corrective Action Preventive Action)
- Out-of-the-Box CAPA (Corrective Action Preventive Action)
- Containment Actions
- Root Cause Analysis
- Resolution Plan and Actions
- Approval for Closure
- Effectiveness Review
- 7-Step and 8D layouts
- Lessons Learned
- Out-of-the-Box Standalone Actions
- Out-of-the-Box Meetings
- Integrates with all other
- CompliantPro modules
Assessments: Structured Method to Find and Fix Gaps
- Assessments
- Gap Analysis/Process Audits/Security Audits
- Groupings via Profiles
- Scheduling and Tracking
- Automatic scheduling of recurring audits
- Version Controlled
- Questionnaires (Integration with Document Control)
- Launch CAPAs (Corrective Action Preventive Action) for non-compliant findings
- On-line and Off-line Findings captureUpload of Off-line Findings
Customer Information - Better Service to your Customers
- Out-of-the-Box Complaints
- Out-of-the-Box Return Material Authorization
- Out-of-the-Box Customer Surveys
Integrates with CAPA (Corrective Action Preventive Action) - Association with Master Data
- Customer Portal
Equipment Calibration & Maintenance - Avoid Quality Problems and Production Delays
- Calibration / Preventive Maintenance
- Out-of-the-Box Calibration Process
- Schedule of Activity
- Capture of Results
- Out-of-the-Box Preventive Maintenance Process
- Schedule of Activity
- Capture of Results
- Controlled Checklists
- Recurring frequency
- Labor and cost tracking
- Integration with CAPA (Corrective Action Preventive Action)
Supplier Information - Better Service from your Suppliers
- SCAR (Supplier Corrective Action Request)/ Supplier Audits/Supplier Evaluations
- Out-of-the-Box SCARs (Supplier
- Corrective Action Requests)Containment Action
- Root Cause
- Resolution Plan and Action
- Closure via Approval
- Effectiveness Review
- 8D and 7 Step layouts
- Associates Master Data
- Integrates with CAPA (Supplier Corrective Action Request) and NCMR (Nonconforming Material Report)
- Out-of-the-Box Supplier Audits and Evaluations
- Equivalent functionality of Assessments Module
Supplier Portal
- Equivalent functionality of Assessments Module
Contact Us
Please write your questions in the following form and we will get to you as soon as possible. We look forward to helping you achieve your goals.
GET TO KNOW MORE OF OUR SERVICES
Proplanner
Proplanner is a leader in process engineering and management software for manufacturers who assemble products. These applications automate, streamline and integrate engineering activities for industrial and manufacturing engineers who design and plan production systems for new and modified products.
